A chemical element is a pure chemical substance consisting of a single type of atom distinguished by its atomic number which is the number of protons in its atomic nucleus. It is the simplest molecule of the oxocarbon family.
In coordination complexes the carbon monoxide ligand is called carbonyl.

. Molecular element atomic element ionic compound molecular compound none of the above. D All atoms of a given element have a constant composition and are different than atoms. We review their content and use your feedback to keep the quality high.
The nucleus is a dense region of positive charge that always contains protons and neutrons. A All samples of a given compound have the same proportions of their constituent elements. A atomic element B ionic compound molecular compound D molecular element E none of the above.
Elements does NOT exist as a diatomic molecule in nature. See the answer See the answer See the answer done loading. By definition A Molecular compound is a compound where the bonding of the atoms is by electron sharing which means that these compounds have covalent bonds.
Carbon monoxide is considered which of the following. Carbon monoxide is considered which of the following. The compound which is molecular compound is Choice A.
It is a covalent compound as it is made up of only non-metals while ionic compounds are formed of both metal and non-metal atoms. Organic compounds not only have carbon bonds but also contain hydrocarbons or carbon bonded to hydrogen. Show transcribed image text Expert Answer.
Fluorine is considered which of the following. Carbon monoxide CO is a covalent compound with simple molecular structure. This problem has been solved.
Which of the following substances is not considered to be an organic compound a. A few types of carbon-containing compounds such as carbides carbonates simple oxides of carbon such as CO and CO2 and cyanides are considered inorganic. How many of each type of atoms are there in the formula NH4C2H3O2.
Molecular compound molecular element atomic element none of the above ionic compound. 83 6 ratings Transcribed image text. N1 H7 C2 O2.
A molecular element B ionic compound C atomic element D. Water does not contain any carbon atom in its molecule H2O. It exist as CO molecules and the carbon and oxygen are held together by covalent bonding.
Fluorine is considered which of the following. We review their content and use your feedback to keep the quality high. Carbon monoxide is a molecular compound.
Ammonium fluoride is considered which of the following. Carbon monoxide CO is a covalent compound with simple molecular structure. Which of the following species is a.
Because any of a large class of chemical compounds in which one or more atoms of carbon are covalently linked to atoms of other elements most commonly hydrogen oxygen or nitrogen. Molecular compound molecular element ionic compound none of the above atomic element. Carbon monoxide is considered which of the following-molecular element-molecular compound-ionic compound-atomic element-none of the above.
Carbon is considered which of the following. It is a molecular compound formed of one atom of oxygen and one atom of carbon. 100 7 ratings Transcribed image text.
It is a key ingredient in many. Experts are tested by Chegg as specialists in their subject area. Carbon monoxide chemical formula CO is a colorless odorless tasteless flammable gas that is slightly less dense than air.
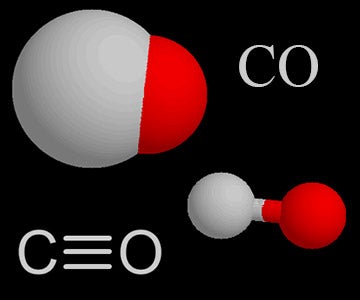
Carbon monoxide is an odorless colorless and tasteless gas. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. Additionally molecular compounds are inorganic and take the form of discrete molecules.
How many total atoms are in the formula Al2 CO33. Florine is considered which of the following. Carbon monoxide CO CID 281 - structure chemical names physical and chemical properties classification patents literature biological activities safety.
Question 6 Carbon monoxide is considered which of the following. C Matter cannot be either created or destroyed in a chemical reaction. It exist as CO molecules and the carbon and oxygen are held together by covalent bonding.
Carbon is considered which of the following. 25 Fluorine is considered which of the following. Carbon is considered which of the following.
Carbon monoxide is considered a compound but carbon and oxygen as elements. Carbon monoxide is considered which of the following. Atomic element none of the above ionic compound molecular compound molecular element.
Experts are tested by Chegg as specialists in their subject area. Question 7 Carbon monoxide is considered which of the following. Which of the following species is a.
Carbon monoxide is considered which of the following. A BaN B Ba_3N_2 C Ba_2N_4 D Ba_2N_3 E none of the above Carbon monoxide is considered which of the following. Which among the following elements does NOT exist as a diatomic molecule in nature.
The oxygen to hydrogen mass ratio of water is always 80 is an example of what fundamental law. While the compound is composed of two or more separate elements. What is the formula for an ionic compound made of barium and nitrogen.
Fluorine is considered which of the following. Carbon monoxide has the formula CO and it is a molecular compound of two atomic elements C and O.
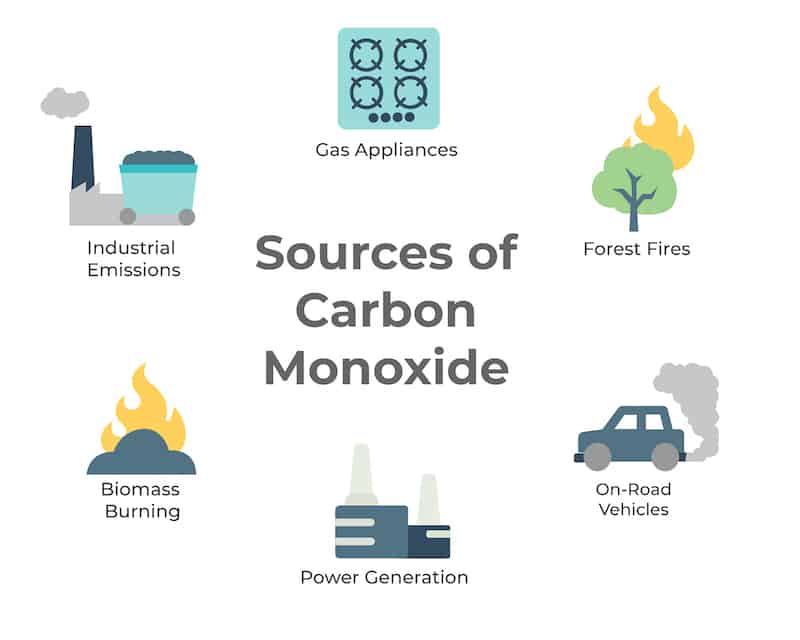
Carbon Monoxide Monitoring Know About Carbon Monoxide Monitoring Oizom
0 Comments